US has seen a small uptick in new COVID-19 instances through the previous week, elevating concern that infections may surge once more.
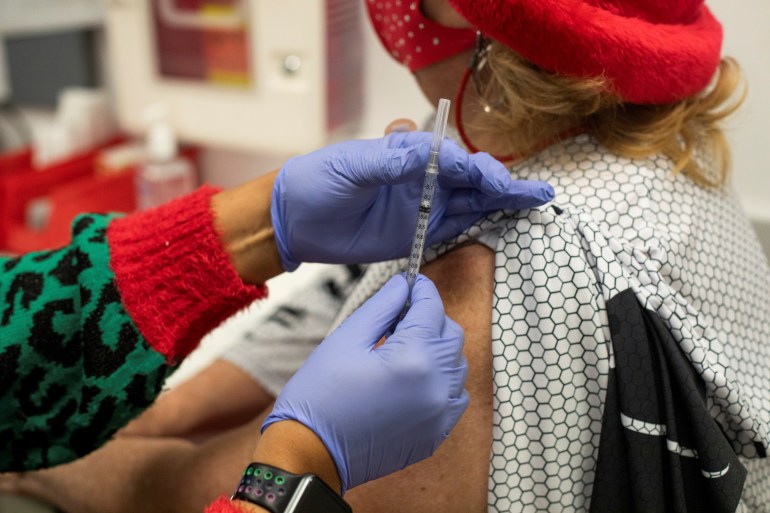
Regulators in the USA have authorised one other COVID-19 booster shot for individuals aged 50 and older, amid considerations that coronavirus infections could start rising once more because of waning immunity to Omicron variants.
The US Meals and Drug Administration’s (FDA) resolution on Tuesday permits a fourth dose of the Pfizer or Moderna vaccines to be administered at the least 4 months after the earlier dose.
COVID-19 instances within the US have dropped sharply since a document surge in January, however have seen a small uptick through the previous week, in accordance with knowledge from the US Facilities for Illness Management and Prevention (CDC).
“Based mostly on an evaluation of rising knowledge, a second booster dose of both the Pfizer-BioNTech or Moderna COVID-19 vaccine may assist improve safety ranges for these higher-risk people,” high FDA official Dr Peter Marks stated in a press release.

Till now, the FDA had cleared fourth doses just for individuals 12 and older who've severely weakened immune programs. The company stated this particularly fragile group can also get an extra booster, a fifth shot.
Two vaccine doses and a booster nonetheless present robust safety towards extreme illness and demise, CDC knowledge present.
Tuesday’s announcement comes at a time of nice uncertainty within the pandemic, as COVID-19 instances within the US have dropped to low ranges after the winter surge however as a sub-variant of Omicron generally known as BA.2 is spreading.
In the meantime, vaccination charges have stalled. About two-thirds of People are totally vaccinated, however half of these eligible for a primary booster haven't gotten one.
Scientists and officers have debated for months if and when an extra booster shot can be wanted as they parsed by knowledge on how lengthy safety from the vaccines and boosters would final.
“It’s not clear that now's the fitting time for individuals to get a fourth dose,” stated Dr William Moss, government director of the Worldwide Vaccine Entry Heart on the Johns Hopkins Bloomberg Faculty of Public Well being.

If there's a surge in instances in late fall or early winter, as is typical for respiratory viruses and influenza, an extra increase could then be wanted, Moss stated. The physique’s neutralising antibodies spurred on by a fourth booster given now could decline in only a few months, he added.
Additionally it is unclear whether or not younger, wholesome individuals will want a fourth shot. The examine of Israeli healthcare employees cited by the FDA prompt that the fourth dose added little extra safety within the age group.
Officers in US President Joe Biden‘s administration have stated that the federal government at present has sufficient vaccine doses to fulfill the demand for an additional spherical of booster pictures for older People, at the same time as funding for the US pandemic response has all however run out.
They stated that except Congress approves extra spending, the federal government probably will be unable to pay for future inoculations, if they're wanted, notably if the vaccines must be redesigned to focus on new variants.

Post a Comment